Technologie Transfer for APIs, Analytics and Production
CDMO-Audit for Manufacturer/Contract-Manufacturer
In/Out Licensingmanagement
Product Launch Management
GMP-Audits for Process-Engineering
Project Management for Technology Transfer und Clinical Studies
Productmaintenance and – development are linked with continuous Improvements in terms of health-related and technology-related challenges. Looking at the scope of product-transfer and new implementation of product development regarding product manufacturing and quality aspects, other processes like license agreements, application filing and marketing management are more or less important to complete the whole technology transfer process. Beside available standards and guidelines for technology transfers, the main focus will be set on the interaction between investments and potential marketvolume for the product’s transfer concept.
Project management support for pharmaceutical manufacturing, quality management and regulatory affairs.
We analyze your current projects, or start with a project idea together with you, set up the necessary project documents with you and your project team, and adapt them to the scope and requirements of your business development.
The project design is based on pharma-specific business processes and workflows, which are combined with classic project management requirements.
The result is project documentation that can be used as a control and governance package for the project sponsor and as a tool and “baseline” for the project manager.
Project Analysis and Setup
With a 2-day workshop with your stakeholders about the project situation of an ongoing or planned project, we can create a project profile to be able to capture and assess all sides as project and process specific as possible.
request free of charge at office@dipha.at, subject: Project Analysis
The workshops are supported by the Association for Life Science Studies CELISS.ORG
Project support for any stage of the project
We can provide support for an entire project or for individual sub-phases. Since different situations may arise in the tension environment during each project phase, additional external help is often useful. Here is a brief list of the individual project phases:
Project development
Design and planning
Project execution
Monitoring and controlling
Project completion
If special know-how is required in one of these project phases, our network of specialists can provide support at short notice.
Request a quote at office@dipha.at, subject: Project Support
Project management integrated in the technology transfer process
In the technology transfer phase, two different organizations usually come together, which must first be coordinated with each other in order to carry out a successful transfer process. This makes the need for a functioning project management mandatory.
Integration in the right place for the stakeholders involved is key to success.
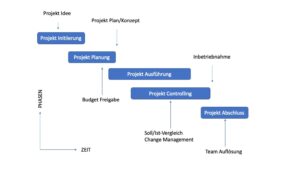
Schematic Project Management Process:
The setup of the project organization and the process are classically done in the structure as it can be directly connected to the technology transfer process. Preparation phases, initiation and execution are strictly adapted in accordance with certified project management programs.
It is essential, however, that the process is completely recorded as a project management process, as this is the only way to integrate the technology transfer process.
Diagram of the technology transfer process:
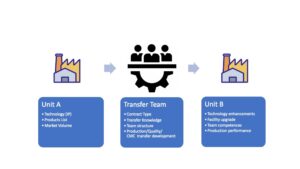
The cooperation form between the transfer partners serves as the basis for the technology process. The constellation of the transfer sender to the recipient establishes the process on its basic pillars. The scope and complexity of the cooperation agreement defines the depth and granularity of the individual transfer steps, ranging from a simple product transfer between contract manufacturers or pure technology transfers between developers of universities to producers of a pure manufacturing company. In between there are all possible variations, but always with the background to fully integrate the pharmaceutical regulatory and GMP requirements according to Guidline ISPE, Technology Transfer Guidline.
Projectmanagement
As different as the technology to be transferred or simply the product is, as multifaceted the team around the transfer can be. Since not only technology but also management is required here, different interfaces come together that have to be coordinated. Experienced teams are confident in managing projects using standards such as IPMA, PMI or similar methods, but also require interface tools such as ECQA for EU specific regulations or project management competence such as Coverdale offers to secure the management level. The framework in which these tasks are bundled typically covers technology transfer in CDMOs.
Teams in accompaniment – We enable people to succeed – together
People are successful when they work together. With its core competence, COVERDALE ® Austria focuses on the soft-skill culture of teams and thus brings the essential topics of leadership and cooperation to the fore. Goal definition, cooperation and conflict management form the central hub for team development. Core competencies are developed through training and coaching. Exemplary 2-day seminars, face-to-face or blended learning packages are used to secure the project management level.
The Project Management Process
We can offer our expertise and experience from technology transfer projects in the following areas:
Project development
Project goal, trigger, definition and description
Project environment analysis
Definition of stakeholders and tasks
Milestones
Structure and planning
Resource planning
Scope definition
Project duration
Cost planning
Preparation of project documents, project packages and templates
Project execution
Interim management on organizational level
Technical support in separate disciplines
Reporting support and project governance
Monitoring and supervision templates
Critical paths
Project budget and tracking
Document management
Milestone Register
Deviation management
Project closure
Closing and project success
Learnings
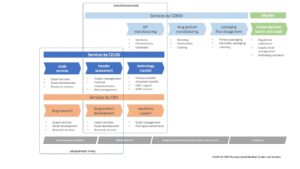
The Process Technology Transfer
Scope and goal
Contract design
NDA, SA, investment agreements
Transfer team
Project structure
Project team
Stakeholders
Planning
Timelines (Stage Gates)
Regulatory
Process Scope (QbD)
Process documentation (PFD, IPC)
Material and substance qualities (API, dosage form)
Specifications
Equipment qualification
Risk analyses
Validations
Cleaning
Analytics Methods
Stabilities
Primary/Secondary Packaging
Trainings
Checklists
Transfer Report
Execution
Budget Management
Project stage gates and packages
Packages DMF, CMC, eCTD
Transfer protocols (API, Analytics, QC)
Execution on plant design
Order management (facility, analytics, API/ingredients, packaging)
Plant validation (process, HSE, IT)
Cleaning runs
Method transfer and protocols
Batch runs and protocols
Validation protocols
Process development reports
Packaging
Stability samples
Verifications
Process
Analytics
Active ingredients
Packaging
Documentation
Final report
Submission documentation (eCTD) package
Validation runs
Budget report
Lessons-Learnt
Transfer report (Stage-Gate)
Project Governance
In order to ensure that the structures, measures and management tools developed are actually effective, project governance should enable control over all processes. This makes it easier to track ongoing adjustments in the project.
The governance package should help you to tidy up your current project or to start new projects in a strategically oriented and target-oriented way.


 English
English